Introduction
The neuromuscular junction is a specialized peripheral cholinergic synapse that provides communication between a motoneuron and a skeletal muscle fiber. Due to its unique structural and functional properties, this synapse is particularly susceptible to a range of pathological conditions, which are outlined in the sections below. Additionally, synaptic transmission at the neuromuscular junction can be influenced by a range of pharmacological agents that exert paralytic effects.
Paralytic Drugs: Nondepolarizing and Depolarizing Muscle Relaxants
Paralytic drugs, also known as muscle relaxants, are commonly used in clinical settings to induce muscle relaxation during surgical procedures and facilitate intubation. These drugs are divided into two major classes: nondepolarizing agents and depolarizing agents.
Nondepolarizing Paralytic Drugs
Nondepolarizing agents work by competitively inhibiting the binding of acetylcholine (ACh) to nicotinic receptors at the neuromuscular junction. By blocking ACh from binding, these agents prevent the depolarization of the muscle cell membrane, thereby inhibiting muscle contraction. Commonly used nondepolarizing agents include:
These drugs generally have a longer duration of action compared to depolarizing agents and are often used for procedures that require extended muscle relaxation. The effects of nondepolarizing agents can be reversed using acetylcholinesterase inhibitors, such as neostigmine or edrophonium. These inhibitors increase the concentration of ACh at the neuromuscular junction, allowing it to outcompete the nondepolarizing agents and restore muscle function.
Depolarizing Paralytic Drugs
Depolarizing agents act as agonists at nicotinic receptors. Unlike ACh, which is rapidly hydrolyzed by acetylcholinesterase, depolarizing agents are not immediately broken down. This leads to prolonged depolarization of the muscle membrane, causing sustained contraction followed by paralysis due to receptor desensitization. The most commonly used depolarizing agent is Succinylcholine (Suxamethonium).
Acetylcholinesterase Inhibitors
Acetylcholinesterase inhibitors, such as neostigmine and pyridostigmine, work by inhibiting the enzyme acetylcholinesterase, which normally breaks down acetylcholine in the synaptic cleft. By preventing the breakdown of ACh, these drugs increase the concentration and duration of action of ACh at the neuromuscular junction, thereby enhancing neuromuscular transmission.
Neuromuscular Diseases: Myasthenia Gravis
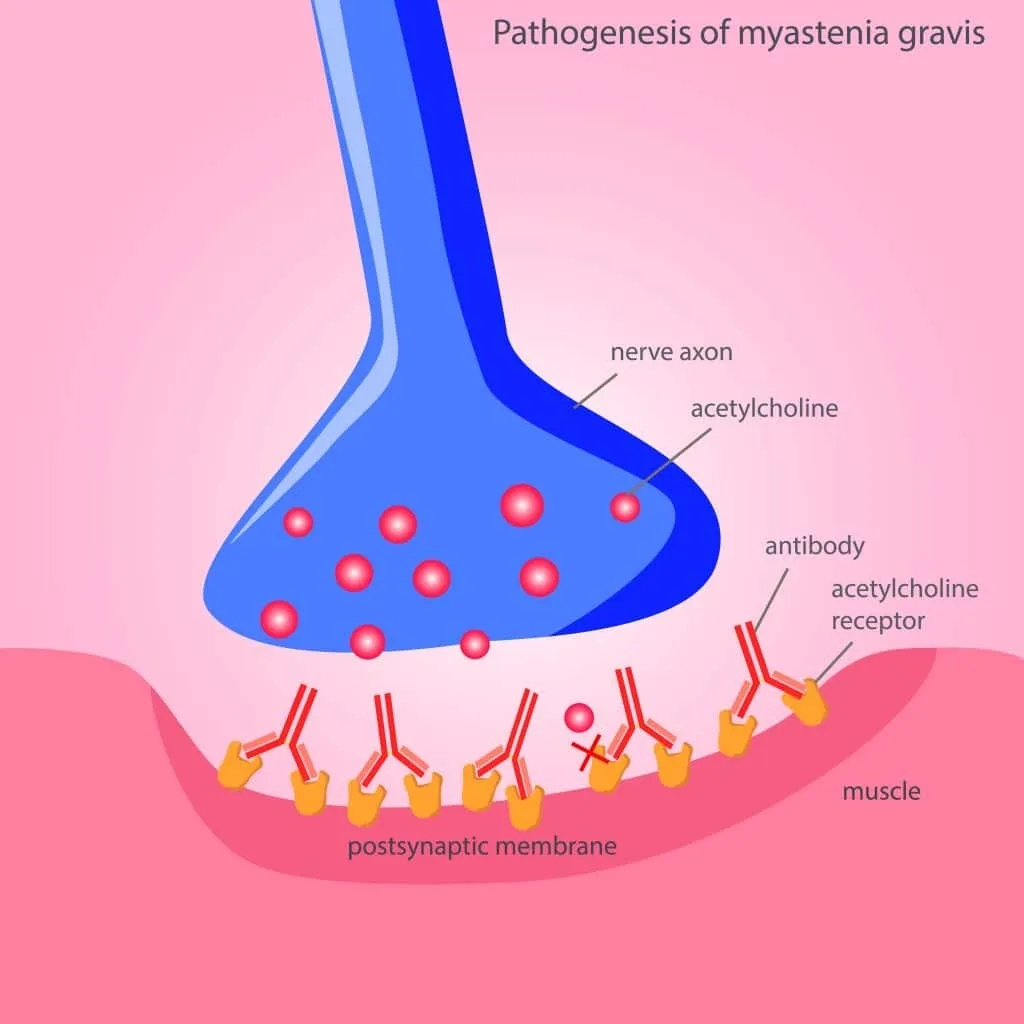
Myasthenia Gravis (MG) is a chronic autoimmune neuromuscular disorder that results in varying degrees of skeletal muscle weakness. The condition is primarily characterized by antibodies directed against acetylcholine (ACh) receptors at the neuromuscular junction, although antibodies against other proteins, such as muscle-specific kinase (MuSK), can also contribute to the disease.
The pathogenesis of MG involves two main mechanisms:
- Antibody-Mediated Receptor Blockade: Autoantibodies directly block ACh receptors, preventing acetylcholine binding and subsequent muscle activation.
- Complement-Mediated Damage: The binding of antibodies triggers the complement cascade, leading to the destruction of the postsynaptic membrane and further reduction in the number of functional ACh receptors.
The hallmark of MG is fluctuating muscle weakness that worsens with activity and improves with rest. This is due to the decreased number of functional ACh receptors and the depletion of acetylcholine in the presynaptic cleft over time.
- Ocular Symptoms: Extraocular muscle weakness often manifests as ptosis and diplopia, as ocular muscles are in constant use and have a lower density of ACh receptors.
- Brainstem Symptoms: Patients may experience difficulty chewing, dysphagia, and dysarthria due to the involvement of bulbar muscles.
- Limb Weakness: Proximal limb weakness is common, affecting the muscles of the shoulders and hips, and may present as difficulty in performing tasks such as climbing stairs or lifting objects.
- Generalized Weakness: In severe cases, generalized muscle weakness can affect respiratory muscles, leading to respiratory insufficiency and crisis.
Symptoms of Myasthenia Gravis typically worsen with sustained muscle use and progress throughout the day. This phenomenon, known as fatigability, occurs as the presynaptic supply of acetylcholine becomes insufficient to compete with the receptor-blocking antibodies, leading to a decrease in muscle response.
Diagnosing Myasthenia Gravis involves clinical evaluation, serological testing (for presence of antibodies against the ACh receptor), and electrophysiological studies (MG patients show a decremental response in muscle action potentials with repetitive stimulation).
Treatments for MG include:
- Long-acting Acetylcholinesterase Inhibitors such as Pyridostigmine (Mestinon)
- Immunosuppressive Therapies, including corticosteroids and non-steroidal Immunosuppressants like azathioprine and mycophenolate mofetil
- Plasmapheresis
- Intravenous Immunoglobulin
- Monoclonal Antibodies, including Rituximab (Rituxan) and Eculizumab (Soliris)
- Thymectomy: Approximately 10% of MG patients have a thymoma, and thymectomy is recommended for these individuals to remove the tumor and alleviate symptoms. Even for those without a thymoma, thymectomy can be beneficial.
Lambert-Eaton Myasthenic Syndrome
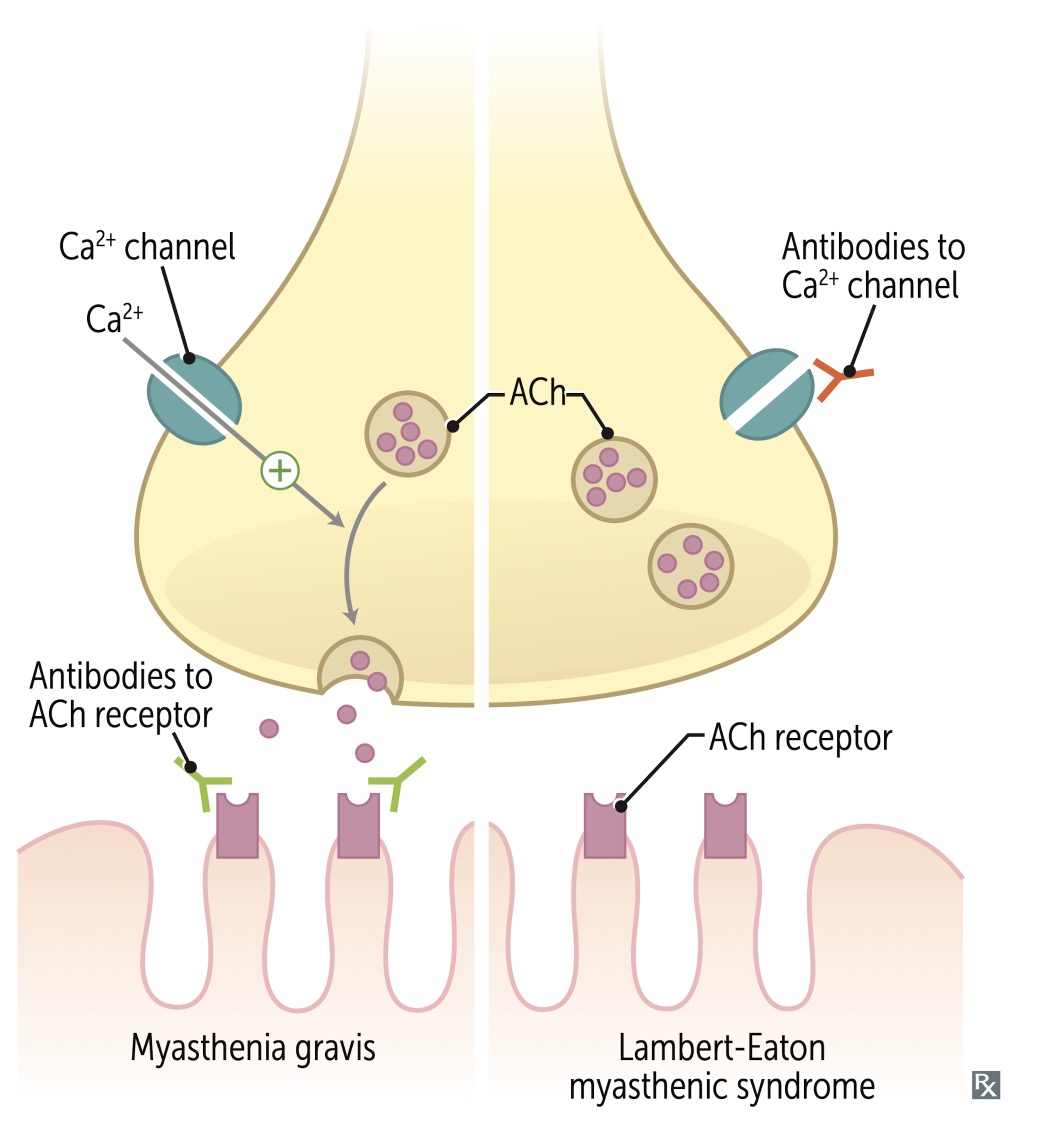
Lambert-Eaton Myasthenic Syndrome (LEMS) is a rare disorder that affects neuromuscular junction transmission, primarily manifesting as muscle weakness. This condition is characterized by the presence of antibodies directed against the presynaptic voltage-gated calcium channels (VGCCs) on motoneuron terminals. These antibodies disrupt the normal calcium flux necessary for the release of acetylcholine.
There are two main types of LEMS: paraneoplastic and non-paraneoplastic. Paraneoplastic LEMS is the more common form and is often associated with small cell lung cancer (SCLC). In this type, the expression of functional VGCCs on the surface membrane of SCLC cells, along with numerous other neural antigens, is believed to be responsible for most, if not all, cases.
In contrast, non-paraneoplastic LEMS occurs without an associated malignancy, and the specific trigger for the development of VGCC antibodies in these cases remains unknown. The clinical presentation of LEMS differs from Myasthenia Gravis (MG) in several ways. Initial limb weakness is more common in LEMS, while extraocular muscle weakness, a hallmark of MG, is less frequently observed.
Treatment for LEMS differs from that of MG. Acetylcholinesterase inhibitors, commonly used in MG, are only marginally effective for LEMS. The primary treatment for LEMS is Amifampridine (3,4-diaminopyridine). Aminopyridines like Amifampridine are beneficial because they block potassium channels, significantly prolonging the depolarization of the presynaptic nerve terminal membrane.
This table from First Aid compares the characteristics of MG and LEMS:
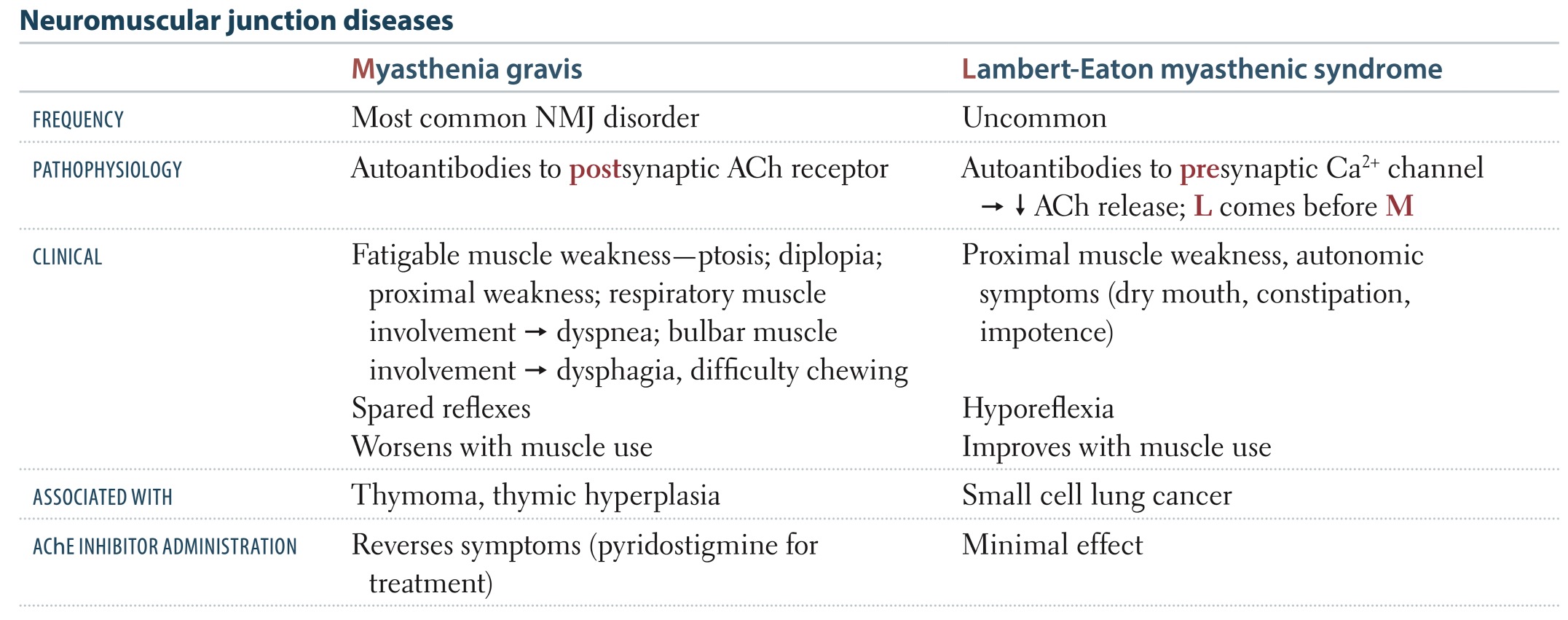
This outline provides a synopsis regarding neuromuscular junction disorders.
Neuromuscular Junction Toxins - Botulism
Organisms of the Clostridium genus, such as C. botulinum, C. baratii, and C. butyricum, are commonly found in soil. These organisms are gram-positive, anaerobic, spore-forming rods that evolved to produce potent neurotoxins. These neurotoxins are responsible for the clinical condition known as botulism, with approximately 200 cases reported annually in the United States.
In 2019, the distribution of botulism cases was as follows: approximately 70% were infant botulism (mainly from consuming contaminated food), 20% were wound botulism, 10% were food-borne botulism, and the remaining 1% were other types.
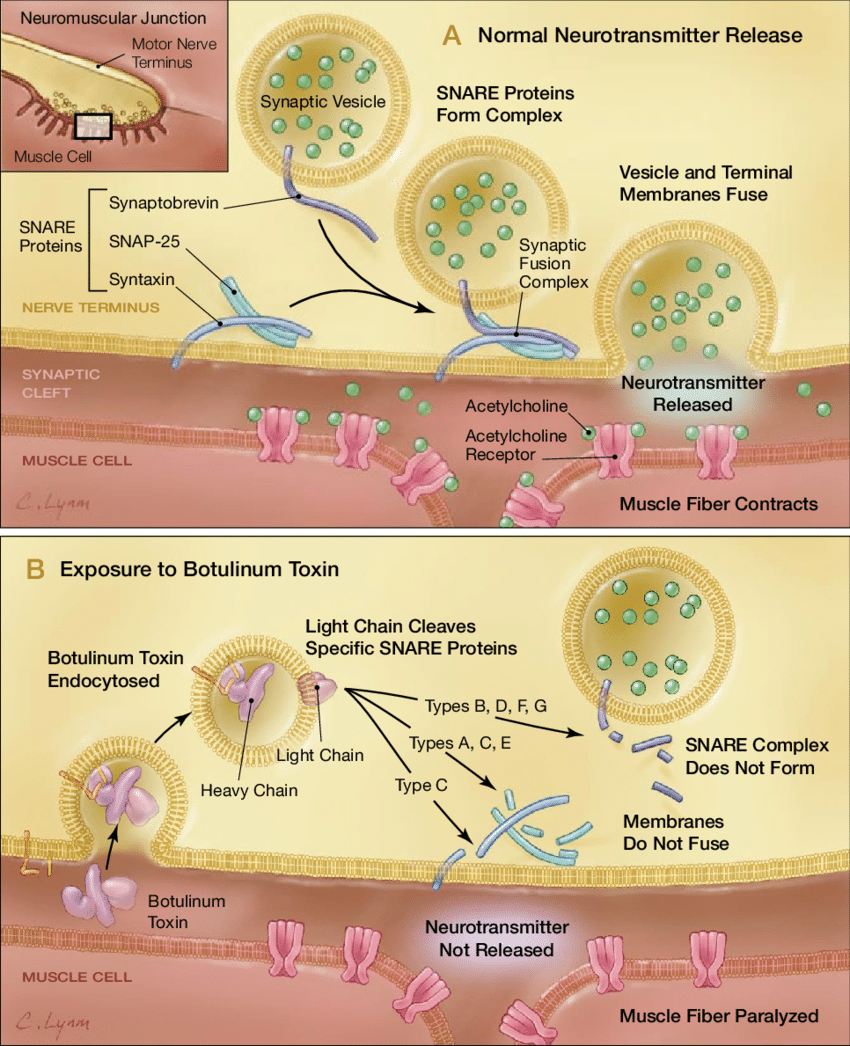
Botulinum toxin exerts its effects by targeting and cleaving key proteins required for neurotransmitter release at the neuromuscular junction, specifically the SNARE proteins. These proteins mediate vesicle fusion with their target membrane-bound compartments. When the botulinum toxin cleaves SNARE proteins, acetylcholine vesicles are unable to bind to the intracellular cell membrane. This prevents the release of neurotransmitter vesicles.